From the Shroud of Turin to climate change – interview with Timothy Jull
What is it that connects the age of the Shroud of Turin with the understanding of climate change? Timothy Jull, visiting professor at the MTA Institute for Nuclear Research reveals the secret.
29 March, 2018
When I first entered Timothy Jull’s office at the MTA Institute of Nuclear Physics, I admittedly expected a sort of “Indiana Jones figure of the natural sciences”, who just happens to spend his vacation time between two groundbreaking discoveries on the gentle plains of Hungary. His name appears in a dazzling variety of topics from the dating of the Shroud of Turin to the investigation of meteorites – so, it’s hardly a surprise that he is also a renowned scholar of climate science.
We didn’t have to talk for long, however, before I realized that for such an impressive resume one needs rather different qualities than a Hollywood swashbuckler. Namely, some openness and a lifetime devotion to a peculiar isotope, called carbon-14.
 Timothy Jull Credit: mta.hu/Tamás Szigeti
Timothy Jull Credit: mta.hu/Tamás SzigetiA delicious timepiece
Wherever we happen to encounter carbon atoms – either by burning gasoline in our car or just having a quick mouthful of our favourite sandwich – they are most probably of the isotope called carbon-12, with a rather stable nucleus made of 6 protons and 6 neutrons (a comparably stable, but far less common isotope is carbon-13). We can safely say that most of the carbon atoms in our sandwich have not changed a bit during the last few billion years.
Sometimes, however, we sink our teeth into a different kind of carbon isotope, which is chemically completely identical to carbon-12, so, for example they can change places in organic molecules. The nucleus of carbon-14, however, contains 2 more neutrons besides its 6 protons and it’s not that stable: give it some time and it decays into nitrogen-14, with a nucleus made of 7 protons and 7 neutrons. However, what really makes things interesting is that in a given period of time each individual carbon-14 nucleus has the same chance of decaying. Based on this, a characteristic half-life can be calculated: the expected time when the carbon-14 content of a sample halves.
For carbon-14 this half-life is around 5700 years, so if we compile a heap out of our laboriously collected carbon-14 in a showcase, six millennia later future visitors will only be able to observe a still decent, but half-sized heap. And things get worse. Wait another 5700 years, and you will find a heap quarter of the size of the original, 57000 years from the beginning: just less than a thousandth.
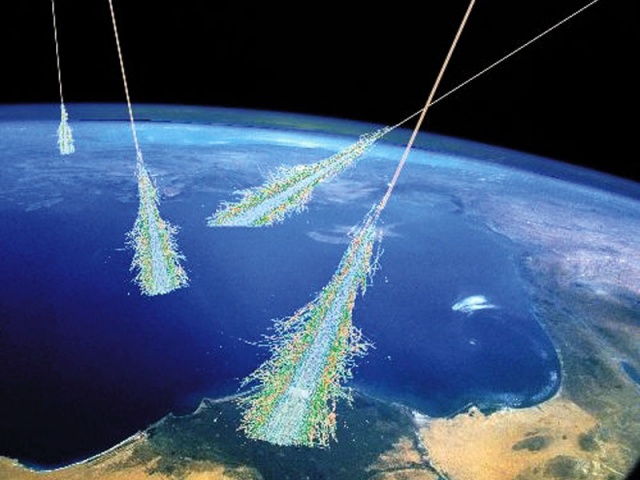 Cosmic rays entering Earth's atmosphere creating a shower of particles Credit: NASA/Simon Swordy (U. Chicago)
Cosmic rays entering Earth's atmosphere creating a shower of particles Credit: NASA/Simon Swordy (U. Chicago)Carbon-14 is created from atmospheric nitrogen bombarded by cosmic ray-induced neutrons. These freshly made carbon atoms quickly react with neighbouring oxygen molecules, producing carbon-dioxide, which in turn enters the cycle of life. If we can assume that the composition of the atmosphere and the cosmic circumstances are constant, an equilibrium is maintained, and a constant portion of carbon atoms taking part in the cycle of life will be carbon-14.
Life is always a wonderful phenomenon, but in our case it is death that comes with exciting news for scientists: after the death of a creature its metabolism eventually stops, and the amount of carbon-14 in its remains “freezes” then starts to fade away according to the rules of radioactive decay. Hence the ratio of carbon-14 to carbon-12 in an artefact gives us clues to the origins of the object — researchers can estimate the time, for example, when flax was harvested for making a piece of cloth, or when a goat was slaughtered for making pages of a codex from its skin. This method is called radiocarbon dating.
Shroud of mystery
British born Timothy Jull graduated from the University of Bristol in 1976 with a PhD in geochemistry, then after doing some research in Europe began his professorship at the University of Arizona, where his main interest became the world of carbon-14 (as well as some other isotopes, see Other isotopes at the end of the article).
This interest lead him to become a member of a prestigious scientific committee, which managed to persuade the custodians of the Shroud of Turin — one of the most popular relics of the Catholic Church — to provide some samples for dating. The first measurements took place in 1988 followed by other independent studies, and the researchers concluded that the fabric definitely originates from the middle ages, so contrary to popular myth, the body of Jesus Christ couldn’t have been wrapped in it.
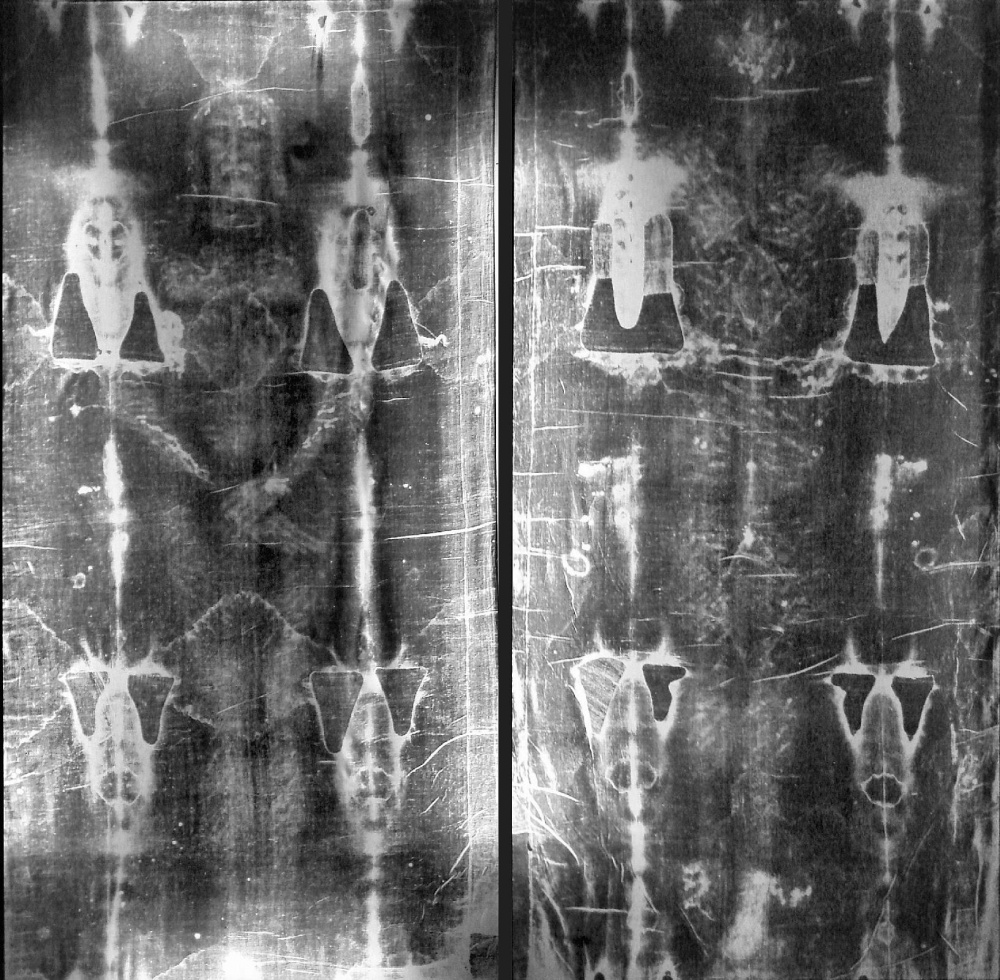 The negative image of the Shroud (two images of the 4.4 by 1.1 metres sized cloth pictured separately for easier viewing) Credit: Wikimedia Commons
The negative image of the Shroud (two images of the 4.4 by 1.1 metres sized cloth pictured separately for easier viewing) Credit: Wikimedia CommonsUnsurprisingly, many doubted the results, however, the Catholic authorities were not among them. The greatest debate unfolded around the preparation of samples: some argued that the small pieces of fabric were taken from a part of the Shroud that was a later addition, others suggested the possibility of contamination by soot particles emanating from the candles or torches illuminating the chamber where the Shroud was kept, and there was a theory of carbon-monoxide chemically reacting with the material. Later studies — one of them conducted by Timothy Jull in 1996 — excluded the possibility of effects like this, so the original dating result is accepted as the most authentic to this day.
Debates like this make one thing perfectly clear: no matter how simple a measurement method may seem to be, the utmost caution is needed to exclude the possibility of measurement errors and bogus artefacts. Caution is even more essential in the case of unique objects such as the Shroud, considering that the requirement of minimal damage during sampling is paramount, hence fierce debates can quickly escalate on the exact location and method of sampling.
The power of relics
The Shroud of Turin was just the first in the line of relics studied by Timothy Jull and his laboratory in Arizona: the trust gained by this successful endeavour opened the path to the radiocarbon dating of other similarly precious objects.
However, one cannot overlook the fact that although the dating of the Shroud of Turin was worthy enough to be published in Nature, many researchers choose to keep a safe distance from objects of this kind. Doing so, saves them from arguing with people, with whom they don’t share any common understanding of scientific facts whatsoever. Taking a more optimistic stance, Timothy Jull believes instead that these relics present a great opportunity for bringing science closer to people. Now we can understand the editors at the National Geographic Society, when they chose the Arizona professor to help them reveal the true history of the Gospel of Judas.
 Kiss of Judas (1304–06), fresco by Giotto, Scrovegni Chapel, Padua, Italy Credit: Wikimedia Commons
Kiss of Judas (1304–06), fresco by Giotto, Scrovegni Chapel, Padua, Italy Credit: Wikimedia CommonsHeretic or true evangelist?
The First Council of Nicea in 325 AD was an important turning point for Christianity as a whole. The Church decided that it was time to end the wild cacophony of gospels (documents summing up the deeds and teachings of Jesus Christ) circulating around, and canonize the authentic ones. The result is well known: words from the Gospel of Mark, John, Matthew and Luke are the backbone of any Sunday mass today.
In contrast however, it’s far harder to bump into the Gospel of Judas, which, understandably, has a rather different take on the passion of the Saviour and the role of Judas in it. The author of this Gospel essentially states that Judas was the true chosen one. He received the true teachings of the Son of God, and the seemingly shameful act of betrayal described so uniformly in the canonized gospels was executed as a direct order from Jesus himself. The crucial question in this case was the document’s date of origin.
After studying the papyrus on which the text of the Gospel was written, Timothy Jull and his co-workers concluded that it was from around 300 AD. Together with the results from analysing the ink by other scientists, it became quite clear that this gospel was among those excluded by the First Council of Nicea. Note that this result does not say anything about the validity or historic accuracy of the contents of the text. However, the rather popular theory of the Gospel of Judas being a forgery created much later, could be safely dismissed.
Useful anomalies
Archaeologists consider the 5700 years’ half-life of carbon-14 quite a fortunate value, because this allows the study of the very period of human history that presents us with most of the artefacts made of organic material (back to some time around 50 000 BC). The studies along this particular timescale on the other hand lead to occasional arguments with creationists, since many of the resulting dates fall into the before-the-creation era, which is, according to their worldview, nonexistent. When arguing against these results they prove to be surprisingly resourceful, from presenting neat collections of erroneous measurement data to questioning the very basics of radiocarbon dating, saying that the ratio of carbon-14 was completely different in the past, so the entire method is corrupted.
Their arguments are usually easily dismissed by any reasonable scientist, however this last argument is not completely unfounded. The ratio of carbon-14 does indeed change with time: human activity in modern times does have a significant effect on the ratio of carbon-14 — that’s why it is quite difficult to do radiocarbon dating on anything younger than 300 years. On a longer timescale, though, physical models ensure us that the method is working properly. These recent anomalies lead us to another line of research by Timothy Jull.
 Flare stack of an oil refinery – ancient fossil fuel deposits contain no carbon-14 Credit: stockfresh.com
Flare stack of an oil refinery – ancient fossil fuel deposits contain no carbon-14 Credit: stockfresh.comTo understand these fluctuations, just consider the fact that since the Industrial Revolution humanity has been using an ever increasing amount of fossil fuels — carbon, petroleum products and natural gas. The carbon atoms in these materials were the part of the bodies of creatures living tens of millions of years ago on the surface of the Earth, so they contain no carbon-14. Hence burning huge amounts of fossil fuels steadily dilutes the atmospheric carbon-14 concentration.
There is another, rather surprising effect, that violently pushes the hand of the carbon-clock in the opposite direction. In the middle of the 20th century, just over the course of a decade aerial nuclear blasts (strong artificial neutron sources) doubled the amount of carbon-14 in the atmosphere. So, in the 1950s the atmosphere suddenly “became younger”, after that, with increasing fossil fuel consumption, it is “growing older” at an increasing pace.
Atmospheric carbon-dioxide readily dissolves in the water of the oceans, where its carbon content accumulates in the bodies of various organisms, or gets depleted in their shells. This way, analysing the carbon-14 content of different habitats helps understanding the details of the global carbon cycle and the effects of human carbon-dioxide emission. Timothy Jull takes part in such an EU-funded climate research program, hosted by the MTA Institute for Nuclear Research, which he first visited about 5 years ago as a Fulbright fellow.
The mysterious rock of Kaba
The town of Debrecen offered other exciting research possibilities, among them the famous Kaba meteorite. The object containing significant amounts of carbon (a so-called CV-chondrite) fell to Earth near the small town of Kaba in the evening of April 15, 1857. This was one of the first space objects that has tested positive for organic compounds. Because space objects are constantly subjected to the bombardment of cosmic rays, isotopes — carbon-14 included —are continuously formed in their material. However, the strength of cosmic radiation decreases the more you go from the surface towards the core.
Credit: Sketchfab/Department of Physical Geography and GIS, University of Debrecen
When an object like this enters the atmosphere of the Earth, its surface heats up, causing it to partially evaporate and partially transform. If we know the time of its entry, with clever sampling, it’s possible to deduce its history before the entry. The Kaba meteorite, according to the study of Timothy Jull, was probably a part of a much greater asteroid — similar to Ceres of nowadays.
After reading all of the above, it’s no surprise that there is a scientific journal dedicated solely to the research possibilities presented by carbon-14. The editor in chief of Radiocarbon — a bimonthly journal published by Cambridge University Press — is none other, than Timothy Jull.
Other isotopes
The world of interesting isotopes doesn’t end with carbon-14, of course. Professor Jull also introduced me to two of his other favourites.
Berillium-10 is created from the collision of cosmic rays with atmospheric oxygen. Rainwater transports it down to the surface of the Earth, and quickly precipitates on the rocks and the topsoil. This remarkable behaviour and the half-life of about 1,5 million years makes berillium-10 a fine tool for measuring the effects of erosion. When we learn about the reconstructed histories of advancing and retreating glaciers, there’s a fair chance that the research leading to these results was based on berillium-10 studies. In Switzerland every glacier has been analysed this way, in the Carpathians the investigations began just recently, with the participation of professor Jull.
The world of iodine-129 is quite different: it signals artificial nuclear activity, since its only possible sources on our planet are nuclear experiments and leakages at industrial complexes where nuclear fuel cells are being recycled. (Another, also exclusively human-made isotope is ruthenium-106, which recently appeared mysteriously in very small quantities— see our article here.) A student of Timothy Jull’s is studying the traces of nuclear experiments in Pacific coral reefs looking for iodine-129.This isotope is also used to follow the dispersion of nuclear waste oozing from the damaged Fukushima power plant. Iodine-129 has a half-life of more than 10 million years, so it’s more suitable for longer term studies than the relatively fast decaying caesium isotopes.
