Hungarian scientist’s fuzzy protein theory becomes part of United States curriculum
Having discovered fuzzy structured protein complexes, Mónika Fuxreiter, Head of the MTA-DE Protein Dynamics Research Group, described a new comprehensive protein model with the creation of the theory of fuzzy structured proteins. The online lecture presenting this theory was accredited by the Accreditation Council for Continuing Medical Education; thus it can be included in the official curriculum of any university in the United States. The researcher, supported by the Lendület (Momentum) Program of the Academy, works at the Department of Biochemistry and Molecular Biology at the University of Debrecen, Hungary.
27 September, 2017
 Mónika Fuxreiter
Mónika Fuxreiter The existence of fuzzy structured protein complexes was first suggested by Mónika Fuxreiter and Péter Tompa in 2008. This new theory contradicts the classic paradigm about how proteins work, according to which there exists a direct relationship between the sequence of a protein and its function. However, fuzzy complexes are generally made up of proteins without well-definable 3D structures. Their versatile structure is also maintained when interacting with other proteins or nucleic acids. The most extraordinary property of these complexes is that conformational diversity might have biological consequences, often including malfunctioning.
The existence of fuzzy protein complexes has been proven through several experiments, which gave way to a deeper understanding of the problem, and was thus developed into a comprehensive theory. The Fuzzy Complexes Database (FuzDB) was created by the MTA-DE Lendület (Momentum) Protein Dynamics Research Group. The database contains a comprehensive list of fuzzy complexes and their properties, and also provides help for researchers.
Fuzziness plays a key role in the formation of protein interaction networks which is a distinguishing feature of tissue, and it is specifically modified in cancerous cell lines. Viral proteins also use sequences in fuzzy regions to fool the regulatory system of the host organism. Fuzzy regions are indispensable for the functioning of oncogenic virus proteins, as these enable the virus to confuse the work of several proteins in the host at the same time. Fuzzy regions play a primary role in the regulation of the life span of a protein, and thus in the defense against tumors. Structural heterogeneity is of key importance in the evolution of proteins. For example, the fuzzy regions of HoxA11 transcription factor had a crucial role in the evolution of placentals (Placentalia): a complex interaction of these regions made it possible for the prolactin gene to turn on owing to gene Foxo1. This process was missing in the evolution of marsupials (Marsupialia).
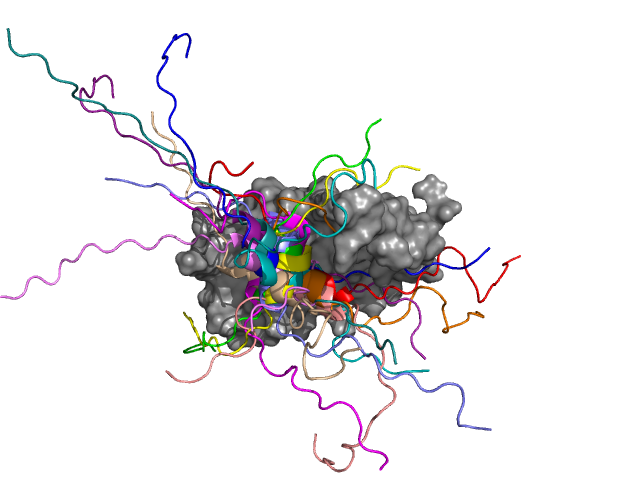 The GCN4 transcription factor (in colour), which regulates the transcription of more than thirty genes, is connecting to the subunit of Med15 (grey) mediator (transcriptional coactivator). The central part of GCN4 takes up the form of a helix conformation, which appears at several positions in the complex. The edges of the helix structures, however, remain flexible. If their dynamics diminish, this affects activity in a negative way. Source: MTA-DE Lendület (Momentum) Protein Dynamics Research Group
The GCN4 transcription factor (in colour), which regulates the transcription of more than thirty genes, is connecting to the subunit of Med15 (grey) mediator (transcriptional coactivator). The central part of GCN4 takes up the form of a helix conformation, which appears at several positions in the complex. The edges of the helix structures, however, remain flexible. If their dynamics diminish, this affects activity in a negative way. Source: MTA-DE Lendület (Momentum) Protein Dynamics Research GroupRegulating the evolution of higher order assemblies
The biological significance of the structural diversity of protein complexes has recently come into the limelight in connection with supramolecular protein complexes. Together with a Harvard scientist, Mónika Furxreiter has shown that the presence of fuzzy sequences is a prerequisite for the higher-order organisation of proteins. These intrinsically disordered regions (IDRs) are present in a number of higher-order structures, from very stable amyloids and prions, to huge signalosomes, to organelles without a cell membrane. The regulatory role of fuzzy regions in the formation, disintegration and physical state can be clearly described. The mutations emerging in protein drops that are created owing to stress and are made up of RNA-associating proteins can in fact trigger the formation of protein complexes or fibres, which might damage neurons. The disease afflicting world famous physicist Stephen Hawking, amyotrophic lateral sclerosis (ALS), is an example of this process.
A comprehensive model of proteins
Fuzzy Protein Theory goes beyond the world of fuzzy complexes, and sets up a general model for proteins: this is a stochastic relationship among the sequences, structure and functioning of proteins. According to this model, these three elements are defined as multitudes among which the rules of fuzzy logic are valid. The multitudes interacting with each other might redundantly produce a similarly structured multitude. In these sets, the probability of the presence of a given population in these emerging multitudes is determined by the circumstances. The functioning of multitudes created by different distributional probabilities might differ qualitatively and quantitatively as well. Consequently, depending on the circumstances (e.g. cell type, outer signal, stress factors), proteins may play different roles. In other words, structural diversity, that is, fuzziness, serves as the basis of adaptation to the environment.
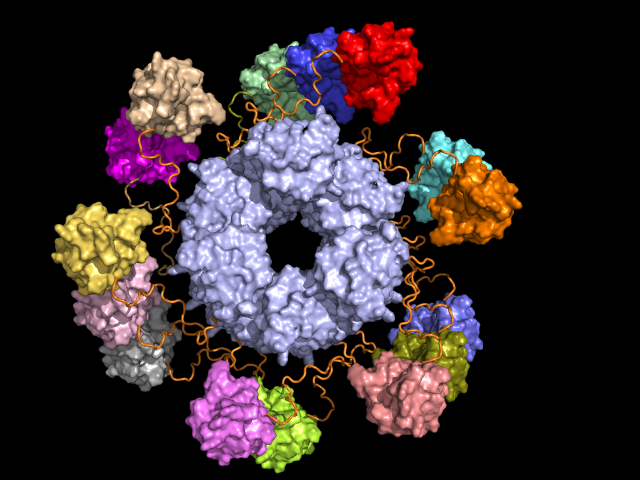 ASC inflammasome, part of our innate immune system, plays a role in the defense against infections. As a reaction to infection, the PYD domain (grey) of the molecule takes up a structure, accompanied by the aggregation of CARD domains (in colour). Dynamic regions (orange) play a crucial role in the formation of huge complexes. Source: MTA-DE Lendület (Momentum) Protein Dynamics Research Group
ASC inflammasome, part of our innate immune system, plays a role in the defense against infections. As a reaction to infection, the PYD domain (grey) of the molecule takes up a structure, accompanied by the aggregation of CARD domains (in colour). Dynamic regions (orange) play a crucial role in the formation of huge complexes. Source: MTA-DE Lendület (Momentum) Protein Dynamics Research GroupThe structural diversity of proteins is now internationally accepted. The interest in the topic is not only shown by publications in journals with high impact factors, but also by a number of lectures delivered at international conferences. Furthermore, in 2017 the Fuzzy Protein Theory was listed among the bioscience lectures of Henry Stewart Talks.
Henry Stewart Talks is an online lecture series of renowned scientists. Alan Fersht and Christopher Dobson (University of Cambridge), Ulrich Hartl (Universität Basel), Eugene Shakhnovich (Harvard University), Lynn Regan (Yale University) and the 2016 Scientist of the Year elected by Science, David Baker (University of Washington, Seattle), are the leading scientists lecturing in this forum. Mónika Fuxreiter, the lecturer on Fuzzy Protein Theory, is the only scientist outside North America and Western Europe in this lecture series. Her talk on the hot topic of fuzziness is among the most visited five percent of lectures on bioscience.
Currently, the MTA-DE Lendület (Momentum) Protein Dynamics Research Group is working on a qualitative analysis of fuzzy phenomena. A predictive method is being created for the identification of fuzzy areas, which makes it possible to predict the dynamic state of the proteins inside a cell. This topic is elaborated in the study being written by Márton Miskei and Mónika Fuxreiter. Computational models make targeted modification of fuzzy parts of proteins, that is, the modification of biochemical processes possible. Presently, the group is concentrating on muscle cell development and the enhancement of immune response given to viral infections.
